Jaundice- An overview
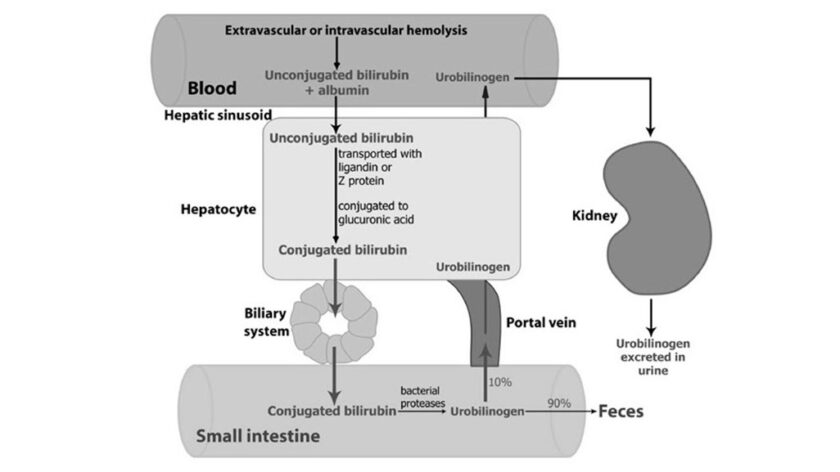
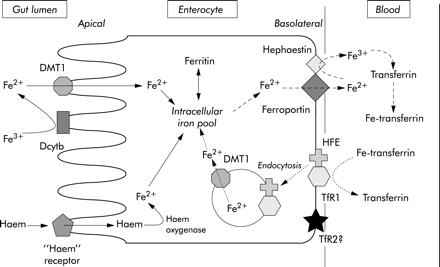
Bilirubin is the end product of heme degradation. From 70–90% of bilirubin is derived from degradation of the hemoglobin of senescent red blood cells. Bilirubin produced in the periphery is transported to the liver within the plasma, where, due to its insolubility in aqueous solutions, it is tightly bound to albumin. Under normal circumstances, bilirubin […]
Jaundice- An overview Read More »